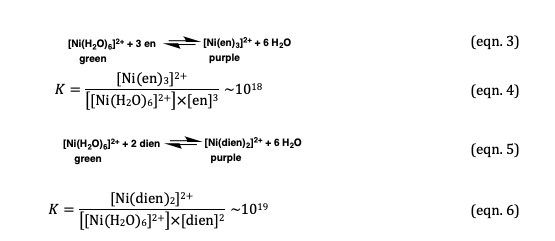Coordination compound: Explain [Co(NH3)6]3+ is an inner orbital complex while [Ni(NH3)6]2+ is an outer orbital complex .
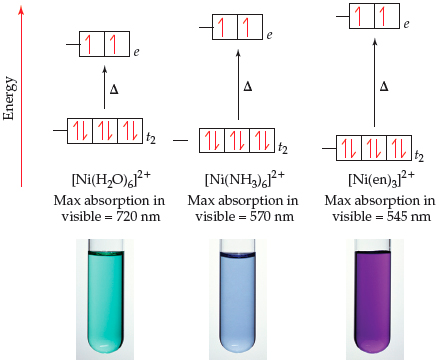
![SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light. SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.
![Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory. Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory.](https://search-static.byjusweb.com/question-images/toppr_ext/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the hybridisation, magnetic property and geometry [Ni(CN)4]2− and [ Ni(NH3)4]2+ using VB theory.

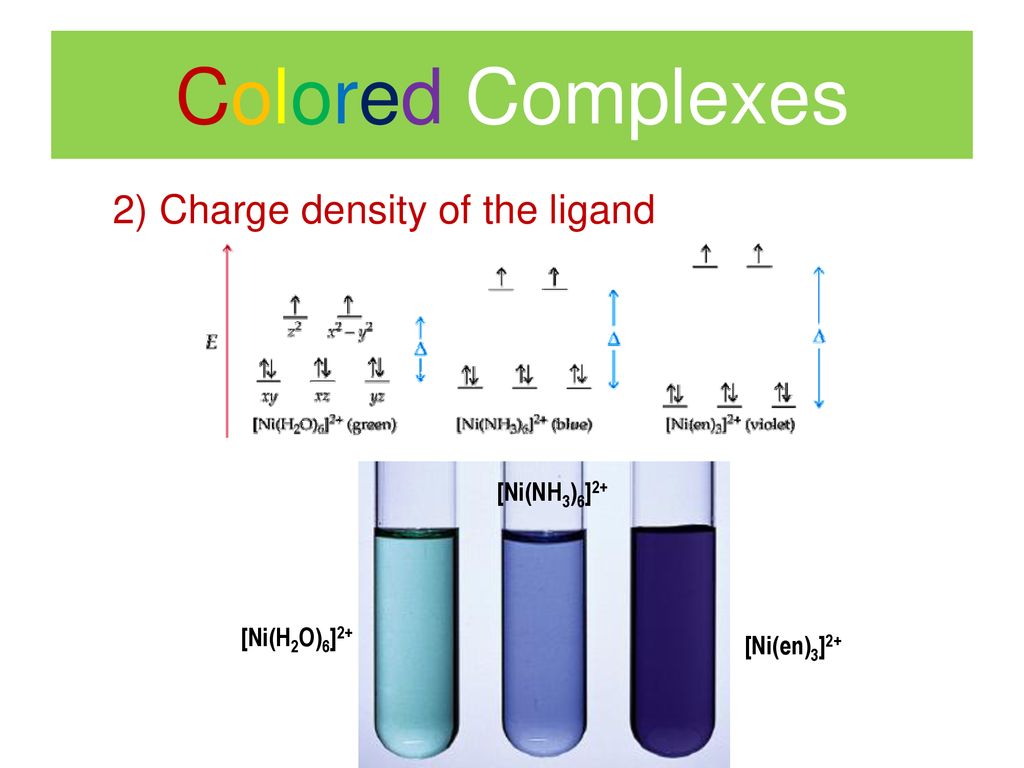
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
![Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube](https://i.ytimg.com/vi/R5RDFu1oYUU/maxresdefault.jpg)

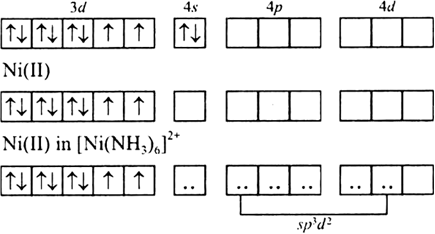
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
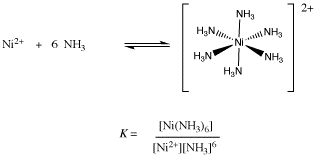
![why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE](https://preview.redd.it/why-cant-ni-nh3-6-2-have-dsp3-hybridization-then-v0-slwfvbv55ria1.png?width=640&crop=smart&auto=webp&s=f4e658df50fc25da185b4ccd9590bfb69fc4c902)
![Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)

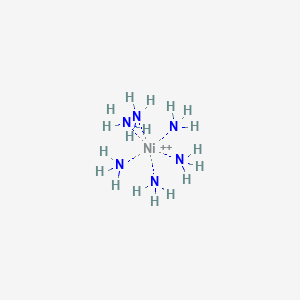
![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
![Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby](https://content.bartleby.com/qna-images/answer/c73807b6-45ed-4420-a3b1-cee7bec70749/203ec893-0265-480e-b058-95e1378e904d/3cda3a30-aca8-11eb-99b8-c16e6153b0d2_IMG_20210504_124206_523~2.jpg)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)