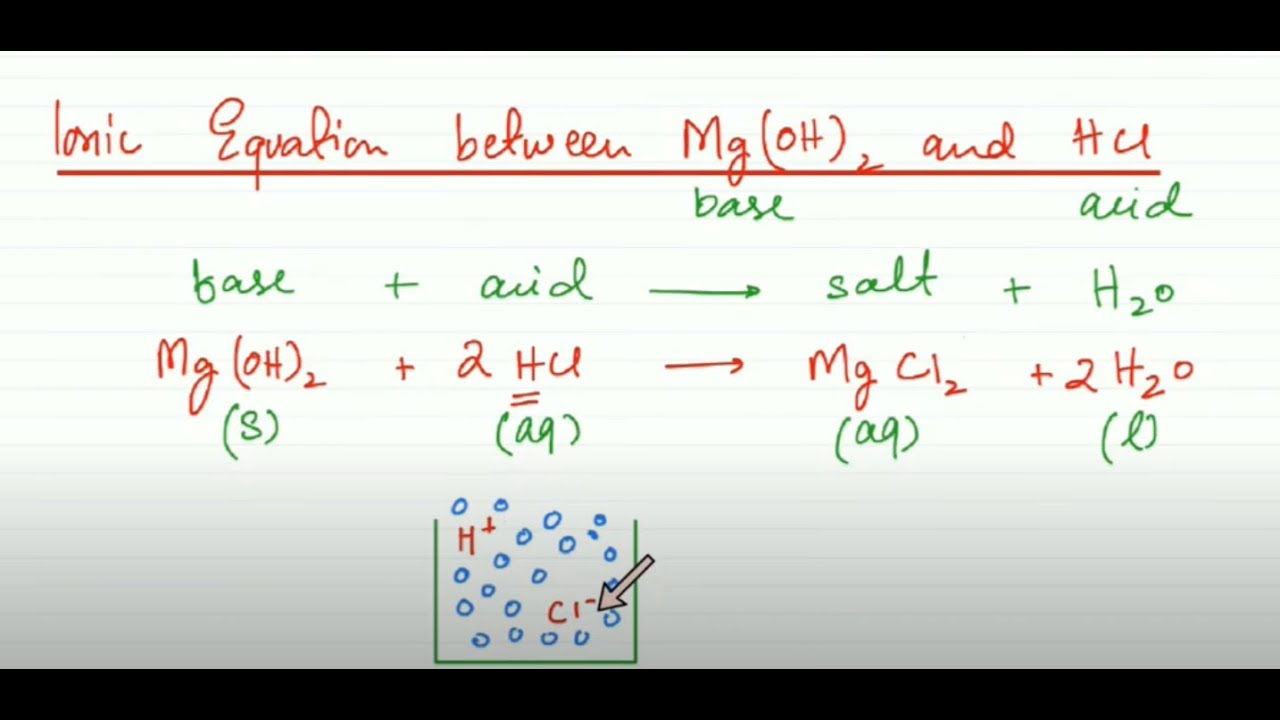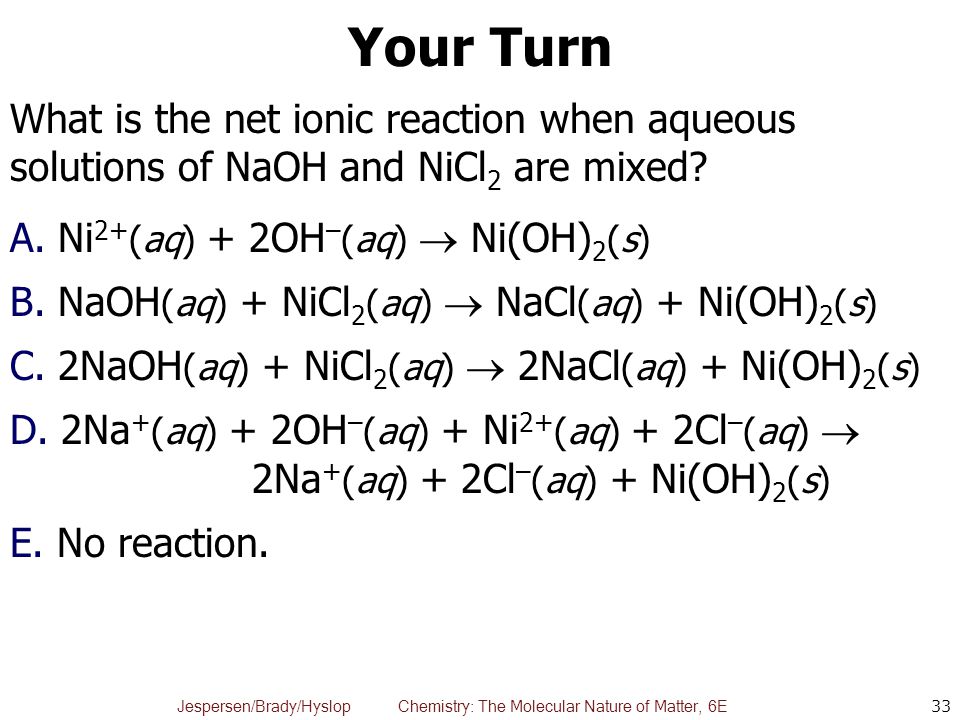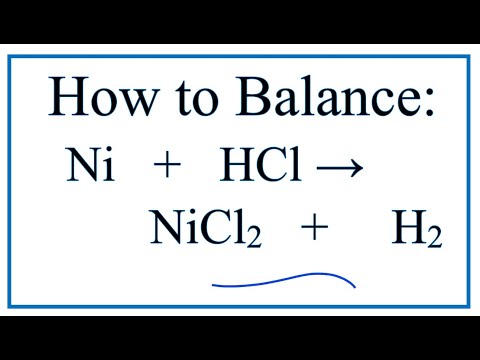
SOLVED: Finely ground nickel (II) hydroxide is placed in a beaker of water. It sinks to the bottom of the beaker and remains unchanged. An aqueous solution of hydrochloric acid is then

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

Electrochemical partial reduction of Ni(OH)2 to Ni(OH)2/Ni via coupled oxidation of an interfacing NiAl intermetallic compound for robust hydrogen evolution - ScienceDirect

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...
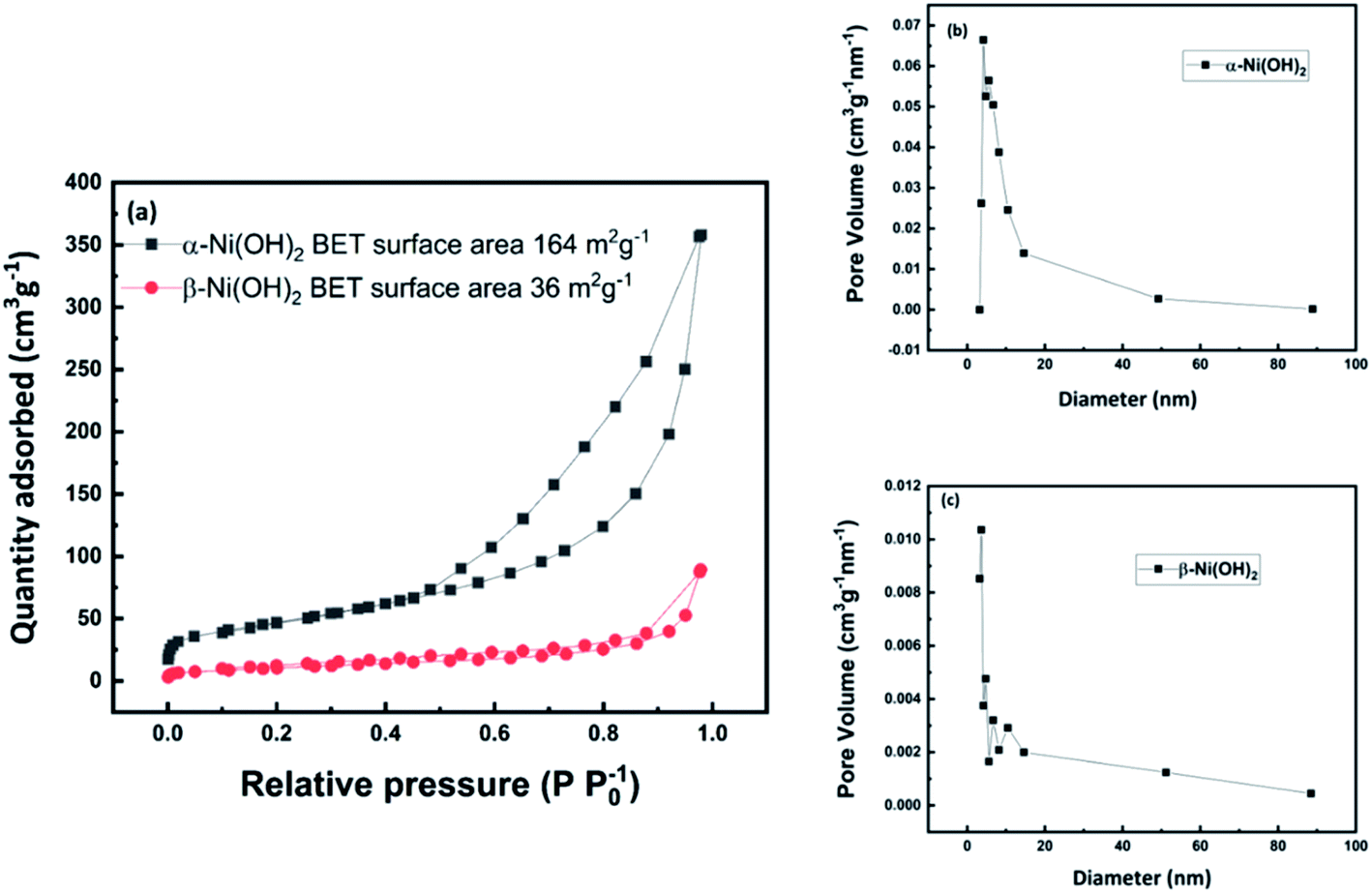
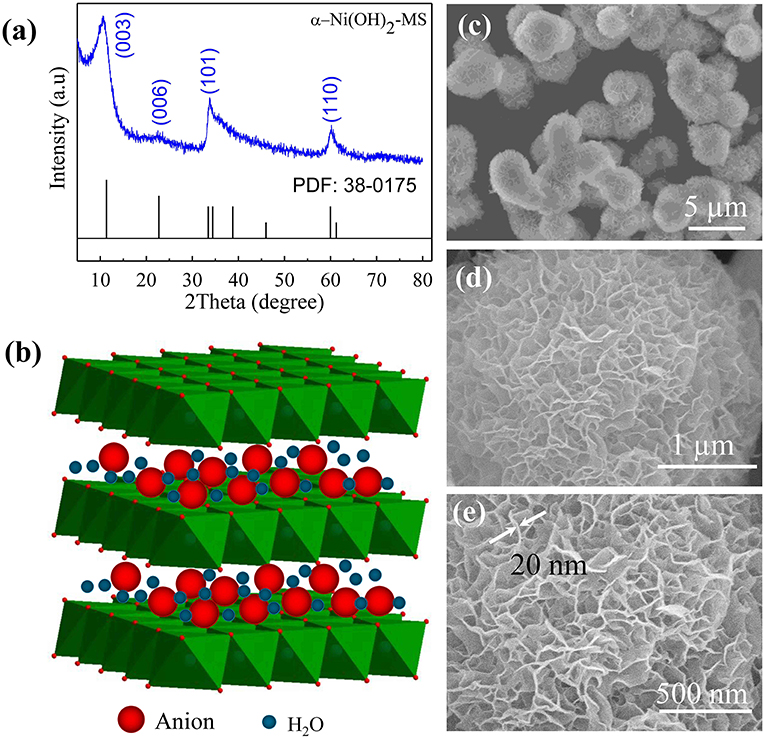
Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis
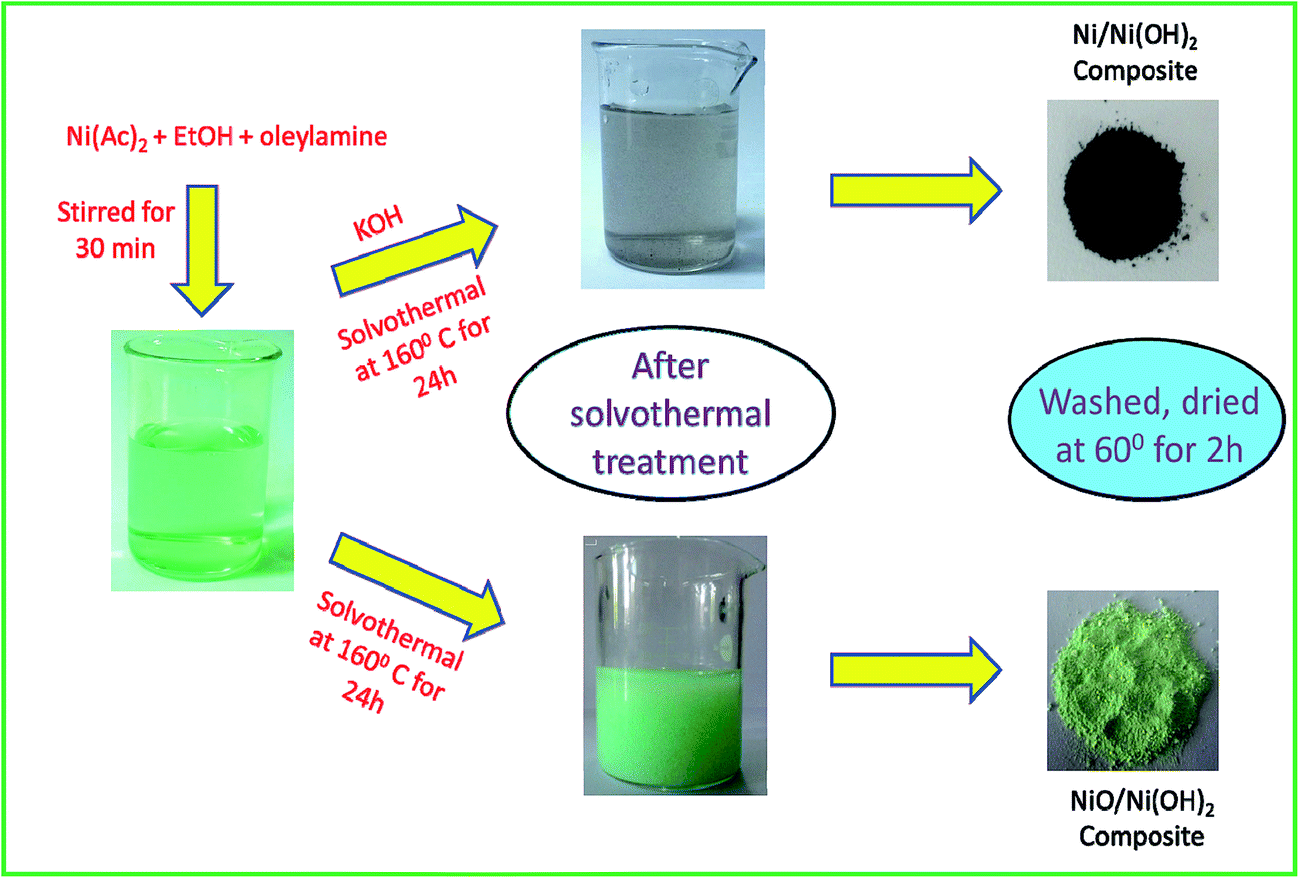
One step synthesis of Ni/Ni(OH) 2 nano sheets (NSs) and their application in asymmetric supercapacitors - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26584G
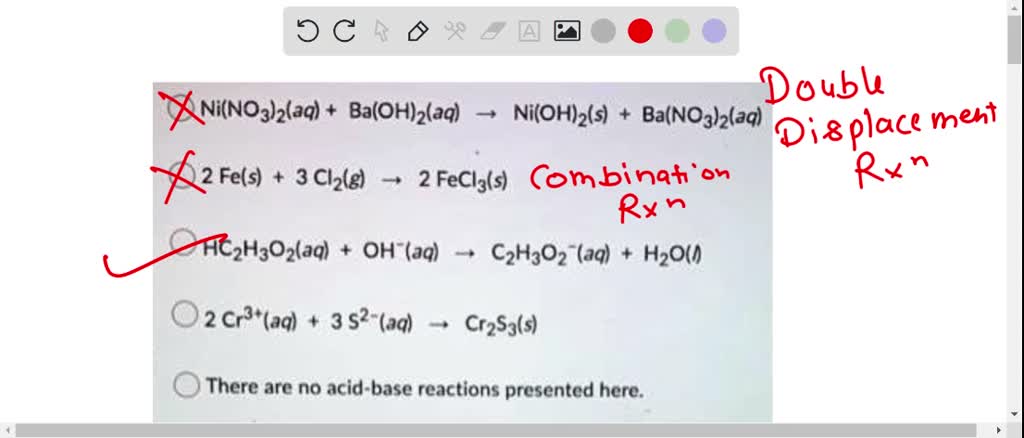
SOLVED: Question 14 (1 point) Which of the following reactions could be classified as an acid-base reaction? Ni(NO3)2(aq) + Ba(OH)2(aq) â†' Ni(OH)2(s) + Ba(NO3)2(aq) 2 Fe(s) + 3 Cl2(g) â†' 2 FeCl3(s)